In Korea, Patent
Term Extension (PTE) system came into effect in 1987. The Patent Term
Extension provision extends patent term up to 5 years for pharmaceutical
product and agrochemicals to compensate patent owners for delays in market
entry due to regulatory review by the Ministry of Food and Drug Safety
(MFDS). It is critical for pharmaceutical companies to cover their R & D
costs by obtaining sufficient compensation from an exclusive market position.
According to the
Korean Intellectual Property Office (KIPO), the 525 applications for
Patent Term Extension has been filed by the end of 2016 since the first
application was filed in 1999. .png)
The number of the KIPO actions for patent term
extension has steadily increased.
.png)
As the approval-patent linkage system like
in US Hatch-Waxman Act was firstly introduced in 2012 and fully
implemented in 2015, PTE has become key tools in the area of protection of
patent rights to either brand-name drug or generic manufactures.
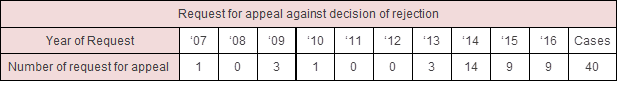
In particular, after
patent listing was implemented in 2015, the huge number of requests for
patent term extension invalidation was filed to obtain 9 months period of the
first generic exclusivity. The first generic company to challenge a listed
patent includes all companies which challenge the invalidation within 14 days
of the first action filed, thus once one generic company files an
invalidation, other generic companies just rush to file the same
invalidations in order to be listed as such “first generic company”.
.png)
|
PTE can be revoked through an invalidation trial before the Patent Court. According to the KIPO, the number of PTE invalidation trials before the Patent Court increased by 57.1% in 2015 and 74.4% in 2016.
As pharmaceutical research and development continues, there will be a corresponding increase in patent litigation. Korea’s Patent Term Extension system has been continuously improved, thus the latest information on the systems and deeper strategies will be needed to branded manufacturers and generic manufacturers.
Should you have any question, please contact us. ※ The information provided on this website is for informational purposes only for our clients and colleagues and is not intended as legal advice. Although we endeavor to keep the information correct, the information may not address all the issues in sufficient detail for your particular needs and may change without notice due to changes in Korean laws, regulations, rules and policies. Visitors to this site agree that KWON & KIM Patent & Trademark Attorneys is not liable for errors or omissions of any of the information provided. Any other reproduction, transmission, distribution, republication or retransmission of such newsletters without the express written permission of KWON & KIM Patent & Trademark Attorneys is prohibited.
KWON & KIM Patent & Trademark Attorneys #713 and #714, Korea Business Center, 309, Gangnam-daero, Seocho-gu, Seoul, Korea Tel: +82-2-586-2019 | Fax: +82-2-587-2019 | E-mail: ip@bspat.com | Website: www.bspat.com |
|
|
|


 | 6762
| 6762
.jpg)